Overview
In this lesson developed by the Arctic Eider Society, students investigate the difference that salt concentration, or salinity, makes to the kind of ice that forms at cold temperatures. Students qualitatively assess the difference in hardness and flexibility, and quantitatively assess the difference in the freezing and melting points to try to unravel the differences between fresh and salt-water ice.
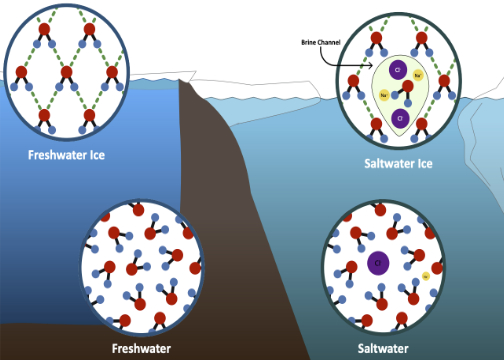
1. Fresh & Salt Water
Students look at fresh and salt water (and ice) from a molecular perspective to understand their different properties.
2. Experiment
Through an experiment where they freeze fresh and salt water, students observe and note these different properties.

3. Reflection
Students are encouraged to further their reflection and apply their knowledge through a series of critical thinking questions.

Resources